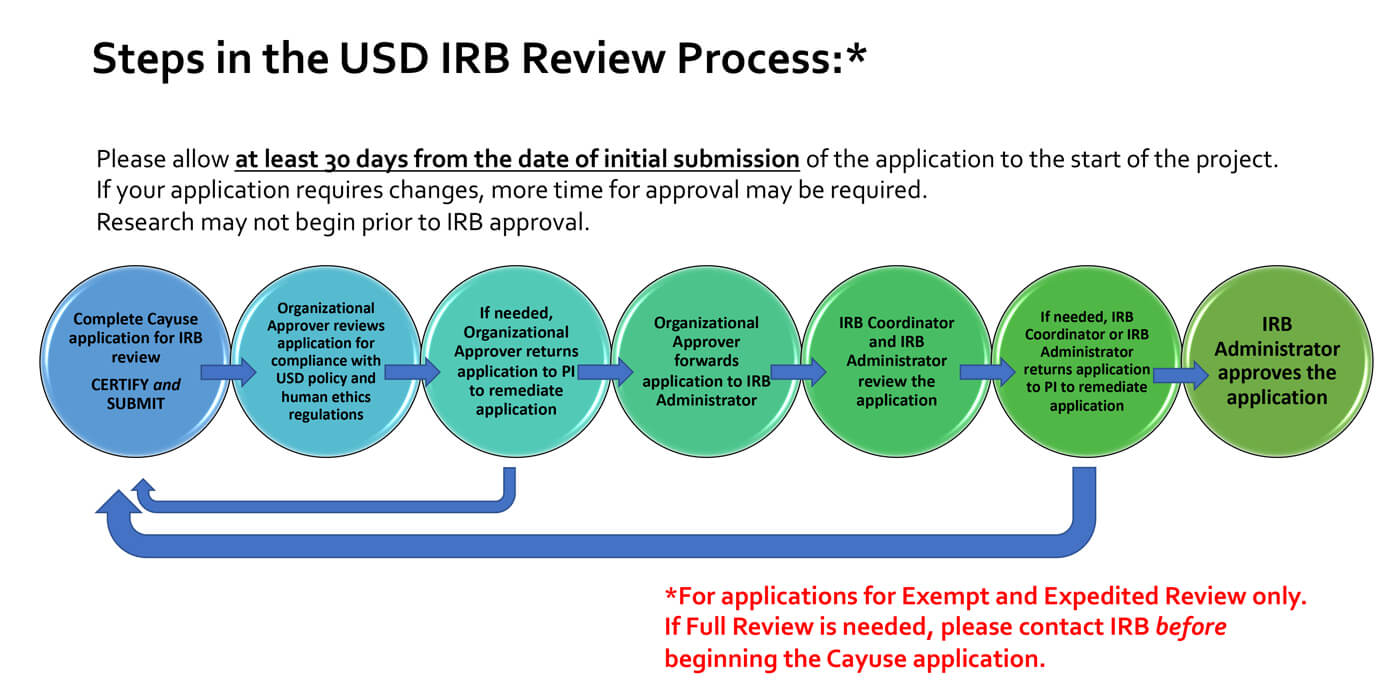
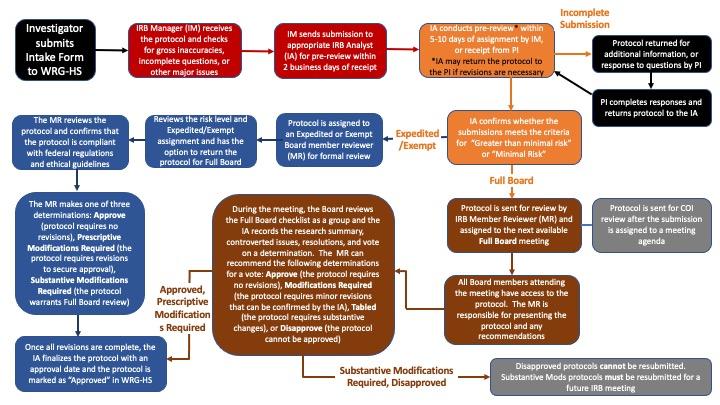
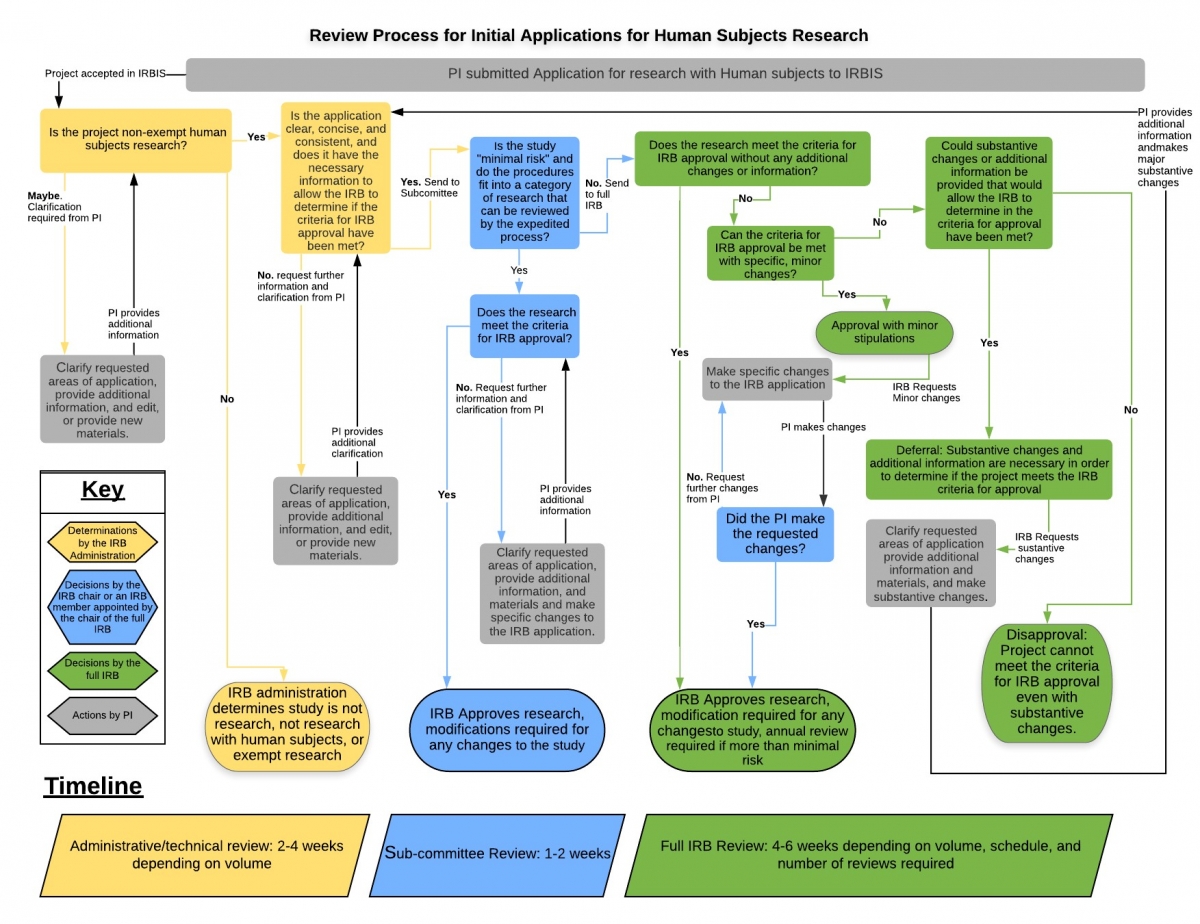
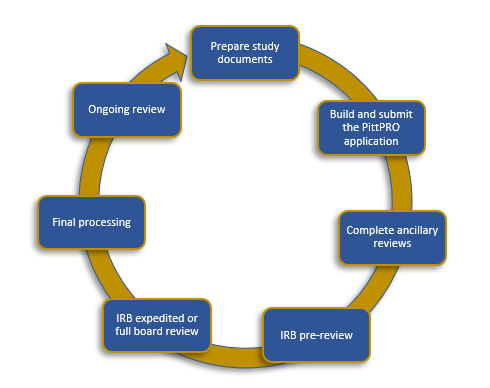
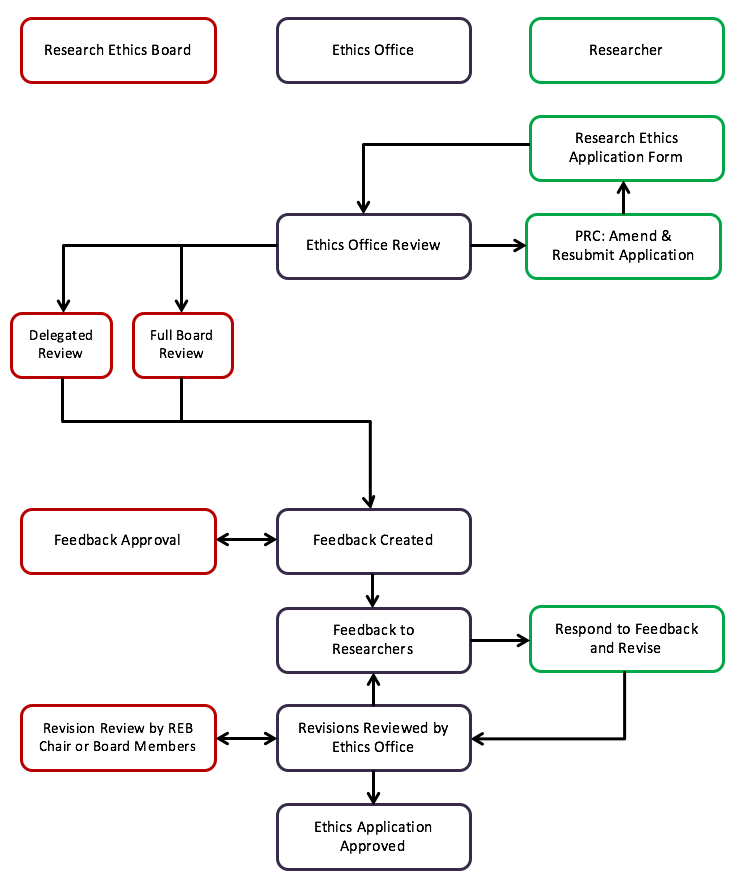
RESEARCH ETHICS BOARD STANDARD OPERATING PROCEDURES SOP Number 403 Initial Review - Criteria for REB Approval Date of Issue Dat
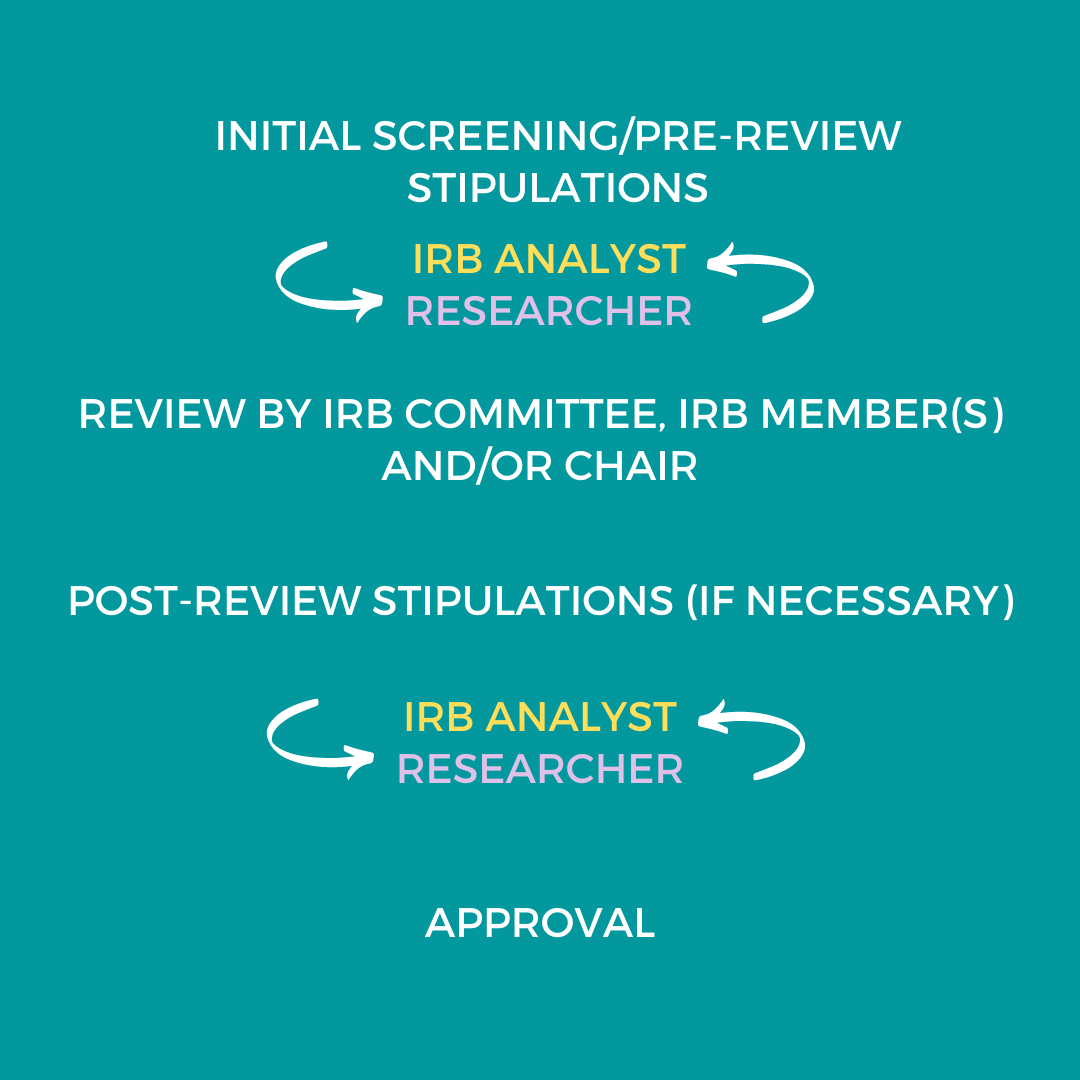
RESEARCH ETHICS BOARD STANDARD OPERATING PROCEDURES SOP Number 404 Ongoing REB Review Activities Date of Issue 2022 07 13 Purp
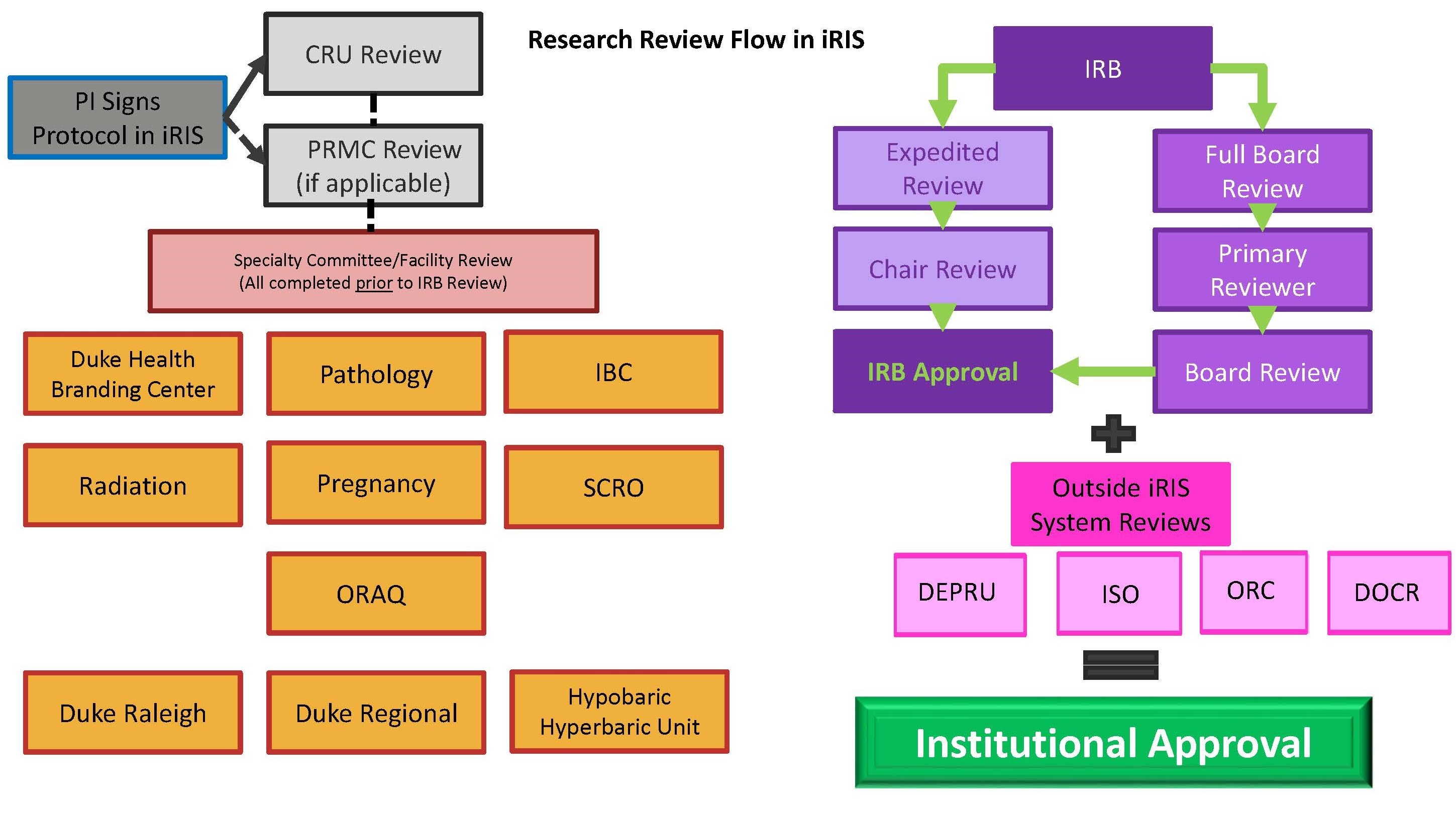
1 University of Prince Edward Island Version: No. 1 Procedure: Research Ethics Board Standard Operating Procedures Associated Po
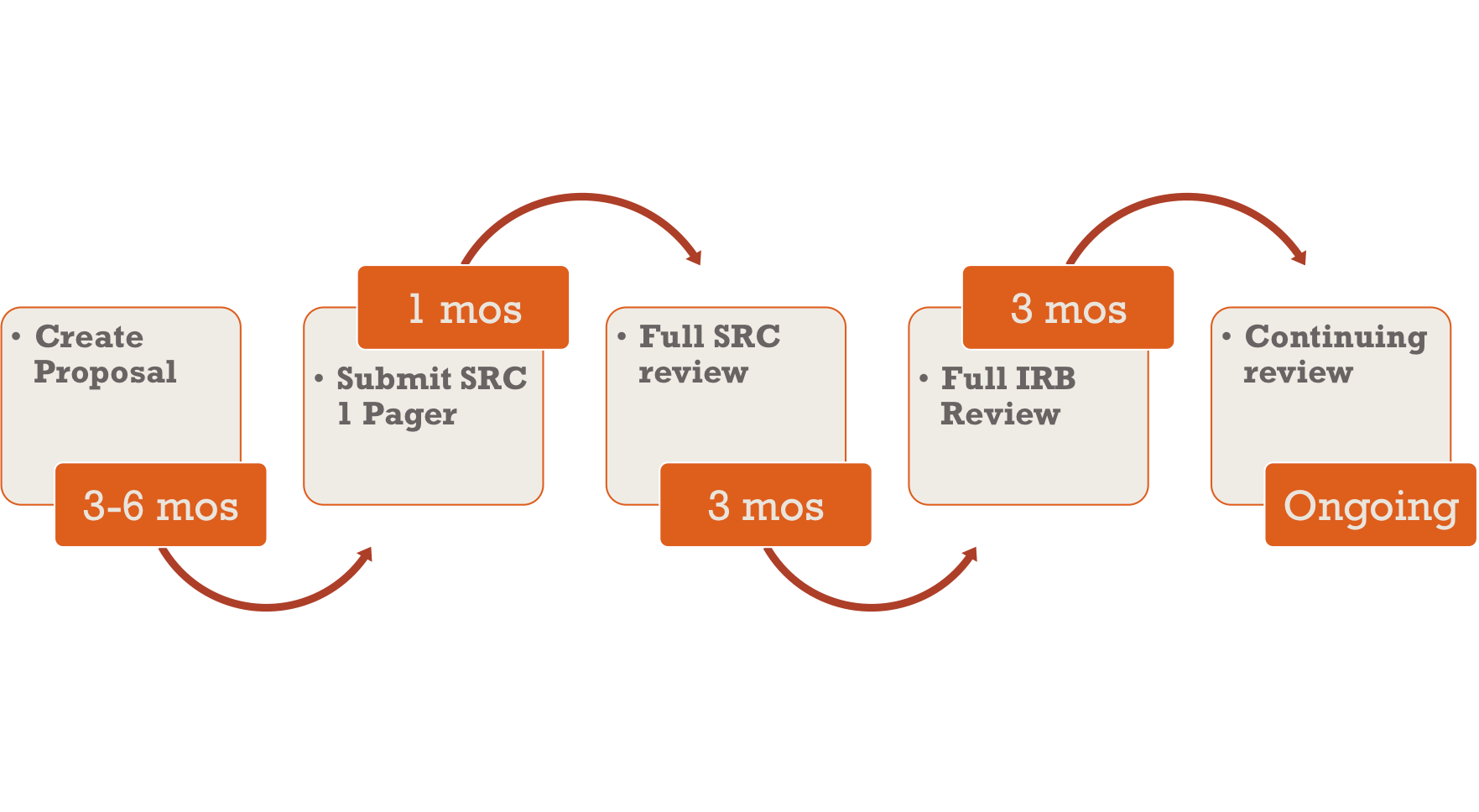
Doctoral Dissertation Research and the IRB | 2021 | IRB Blog | Institutional Review Board | Teachers College, Columbia University
RESEARCH ETHICS BOARD APPROVAL FORM Please check one of the following options before completing the rest of the application. Thi